All
Publications
Insecticides for Mosquito Control: Improving and Validating Methods to Strengthen the Evidence Base

Good decisions require good data. Efforts to eliminate vector borne diseases rely heavily on the use of vector control tools, and the toolbox available to combat insect vectors of disease is growing through improvements to existing approaches and new emerging technologies.
The decision to deploy new vector control tools or approaches on an operational level should be supported by robust entomological evidence to demonstrate efficacy, comprising data collected using appropriate and validated methods. A strong evidence base can also guide effective operational deployment decisions.
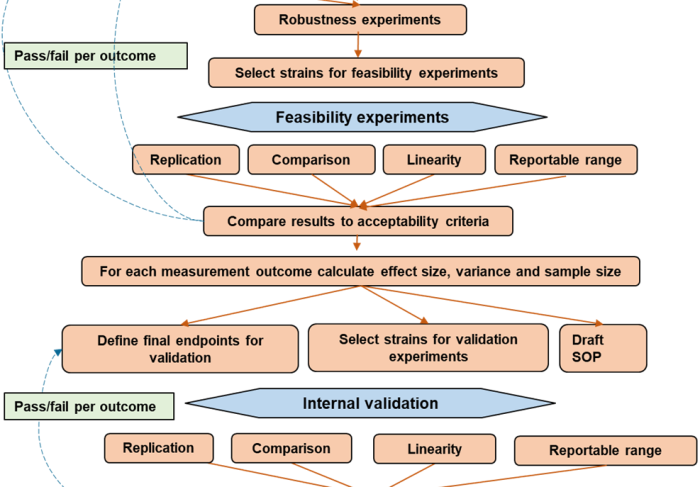
The Insects Special Issue “Insecticides for Mosquito Control: Strengthening the Evidence Base” presents original research into developing and characterising new vector control products, as well as understanding and monitoring insecticide resistance. Review articles explore the impact of insecticide resistance and offer guidance on insecticide choice in the face of pyrethroid resistance. Consensus methodologies are presented, in the form of standard operating procedures designed to be adopted and used to generate reproducible data that can be compared across and between studies.
The editorial states that there is an impetus to better validate entomological methods and that there is a clear need for new and improved methods. Using methods which are fit for purpose is crucial to enable regulators, procurers and implementers to assess and compare the range of tools available to make informed decisions on how best to utilise limited operational budgets. It also highlights the importance of relating laboratory and semi-filed tests to product performance, and emphasises the need for pre-emptive method development and validation.
Driving improvement in method validation and evidence generation is critical. The establishment of the World Health Organization’s Prequalification of Vector Control Products (PQT/VCP) process, and the welcome focus on a regulatory approach to product evaluation, offers the opportunity for a fundamental change in the way we view vector control products. The promise of this new process is that product developers can generate data that reflects the performance of their product, rather than developing products to meet a rigid set of data requirements.
Validation of methods is crucial to ensure that results are reproducible and informative. Currently, data generation for vector control tools is centered around access to the market with a focus on a WHO policy recommendation and WHO PQT/VCP listing. These are important milestones, but data is needed throughout a product’s lifecycle to inform on performance and aid deployment decisions, particularly when implementing resistance management strategies.
We recommend that a comprehensive package of data is generated for a vector control product that goes beyond market access and encompasses lifecycle management. This should include a package of validated methods for generation of the data throughout a product’s lifecycle, and a means for interpretation to assist decision making for implementation. Some of this information will be available through established evaluations (e.g. WHO PQT/VCP), but others should be considered in addition to those requirements to ensure streamlined uptake of new tools. The recommended package of information, and associated data collection and interpretation methods, for a product comprises:
• Scope of the product
• Entomological mode of action
• Intended entomological endpoints
• Regeneration time
• Insecticide content and formulation
• Residual efficacy (bioefficacy over time, physical durability, resistance monitoring)
• Interaction with existing tools or active ingredients.
The pipeline for new vector control tools has never been richer, with a variety of product types and vector control strategies under evaluation. This pipeline is an achievement to be celebrated, but all of these approaches will require testing methods to measure their efficacy and predict or directly determine entomological and epidemiological impact. In all these areas we recommend that the same considerations be taken in developing and validating the required standardised testing methods, including clearly defining the relevant endpoints, standardising or characterising inputs and testing parameters, and being clear on how to analyse, interpret and report data in order to use the results to make robust, evidence-based decisions.
“It is hoped that this Special Issue offers inspiration and guidance on how consistent data can be generated to inform more effective development, evaluation, and use of new and existing vector control tools.” – Dr Rosemary S. Lees
Access to the Editorial “Insecticides for Mosquito Control: Improving and Validating Methods to Strengthen the Evidence Base.” Rosemary Susan Lees, Christen Fornadel, Janneke Snetselaar, Joe Wagman, and Angus Spiers.
Access to the Special Issue “Insecticides for Mosquito Control: Strengthening the Evidence Base”